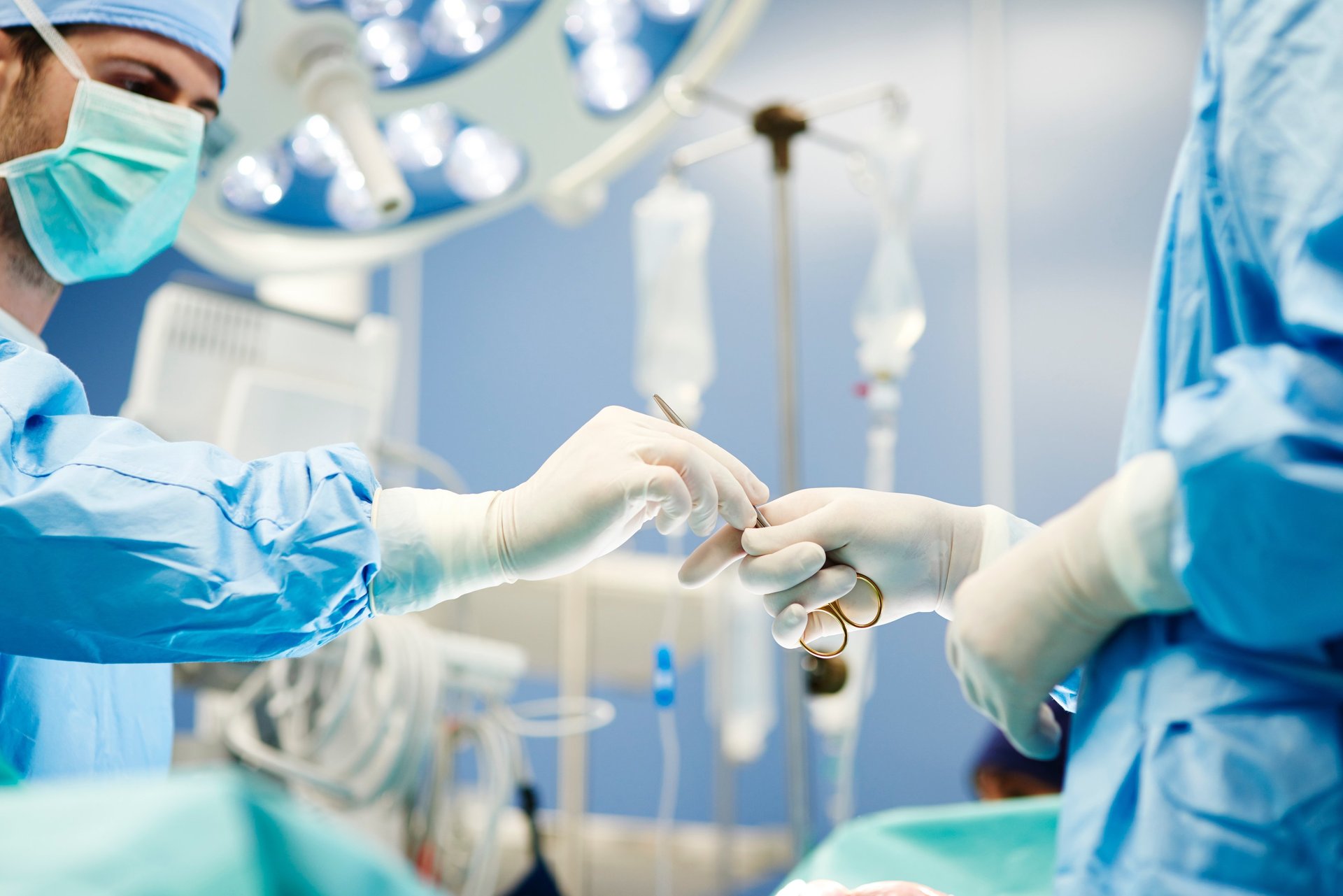
Medical Devices
We have over 25 years experience in helping medical device manufacturers design new products. Our user-centered design process spans the development cycle to help ensure new products have user interfaces that are safe, effective and enjoyable to use.
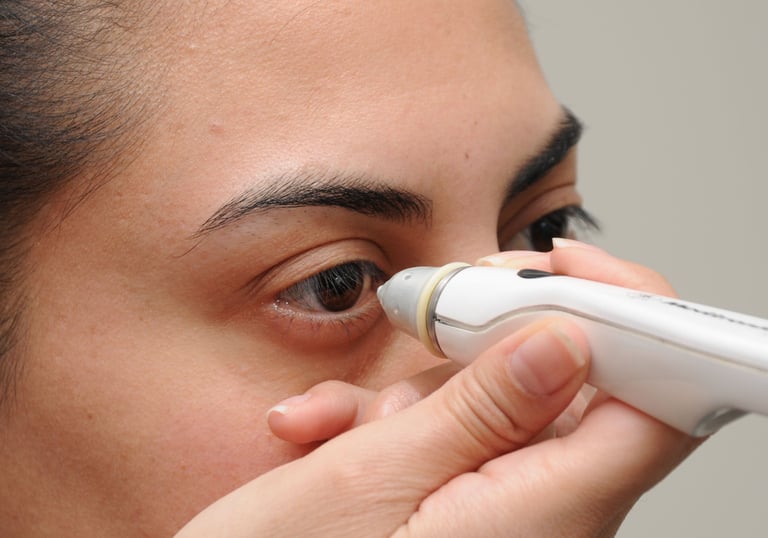
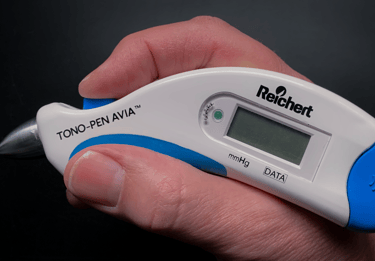
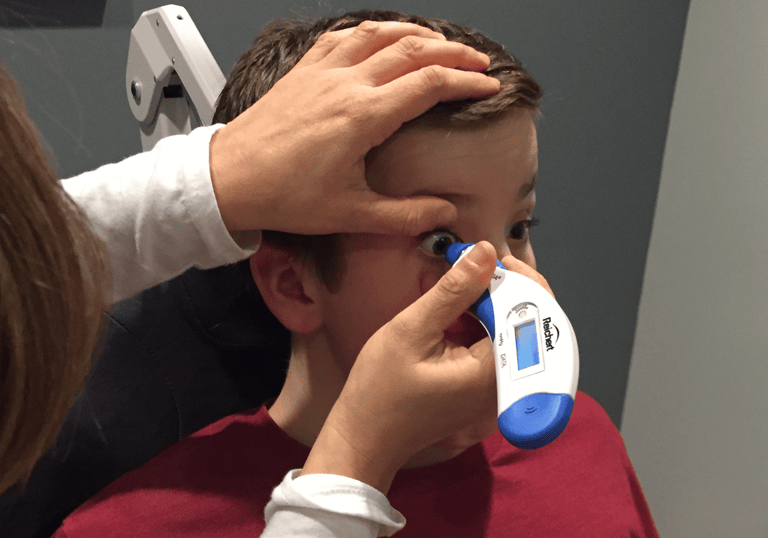
We don’t do focus groups. We believe that real understanding of user needs comes from observing people’s behaviors, interactions and use patterns. We go to OR’s, clinics, labs & homes to understand the use environment and observe how products are really used.
Research & Analysis
We work alongside internal development teams to conceptualize and develop new hardware and software user interfaces. We continually advocate for the user throughout the process and make sure their needs are not forgotten when trade-offs are made.
Design
Evaluation
We conduct formative and summative usability evaluations for medical devices and preparing the usability file and human factors report for FDA review. We know exactly what the FDA expects and help de-risk the submission.
Our work experience spans a wide variety of medical device product types & categories.




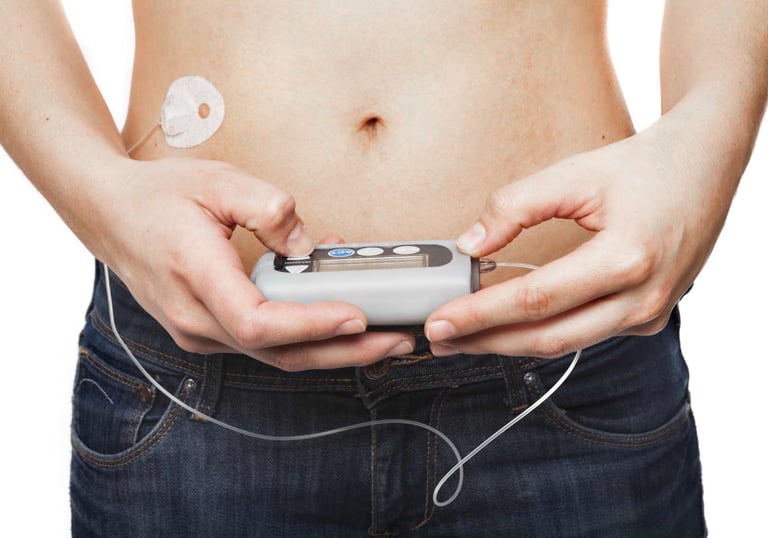



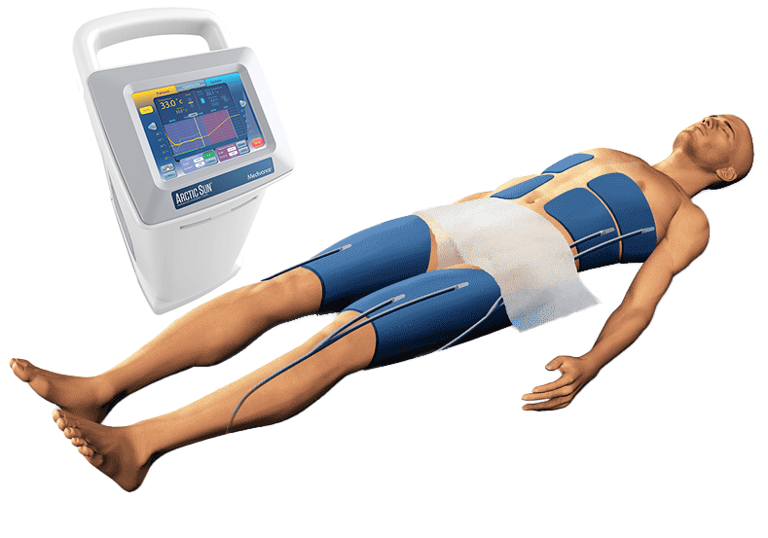
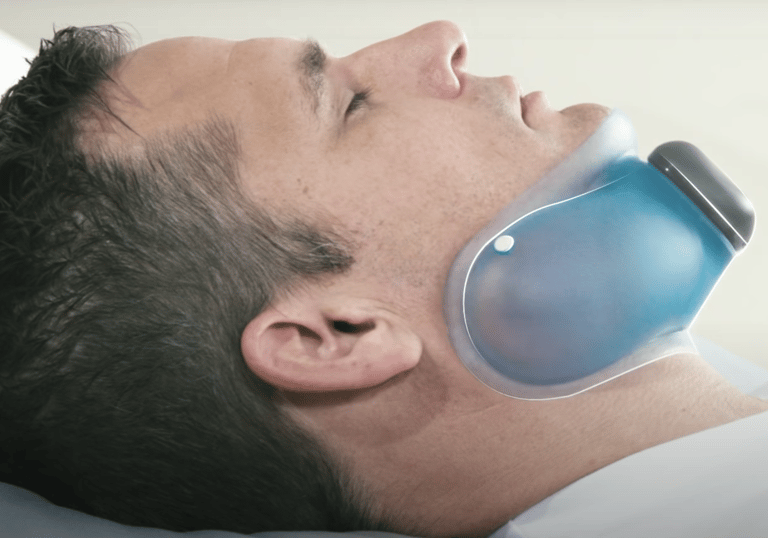
Temperature management systems
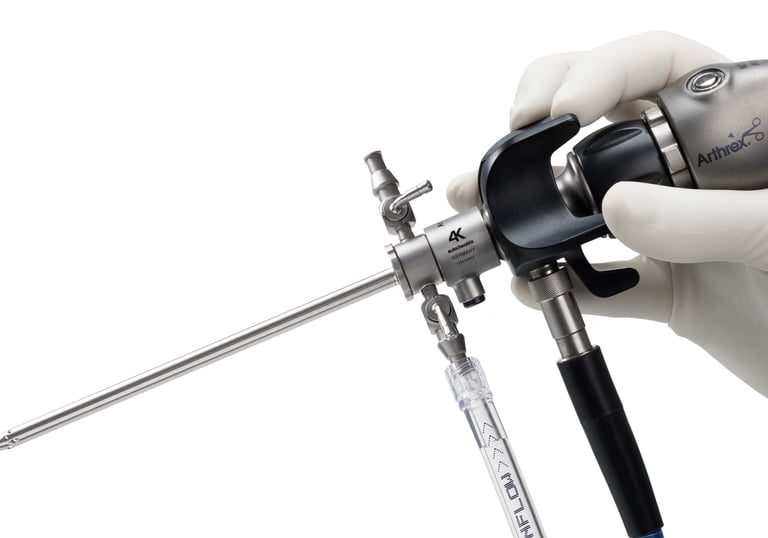
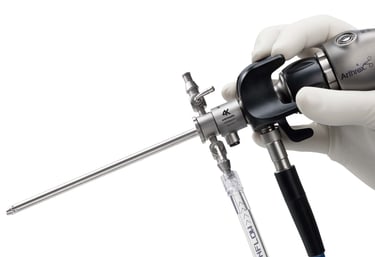


Endoscopic surgery visualization
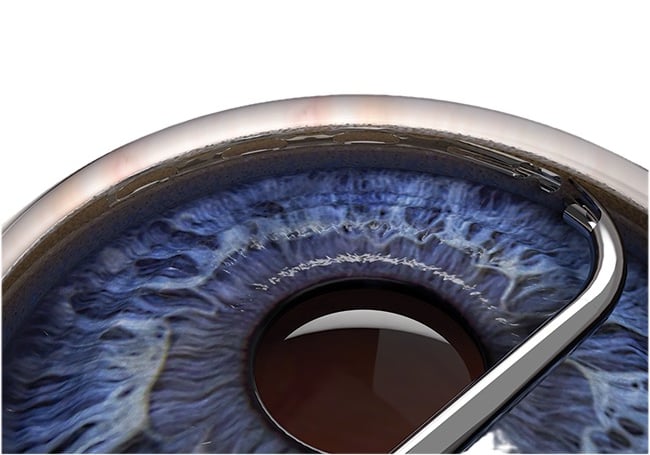
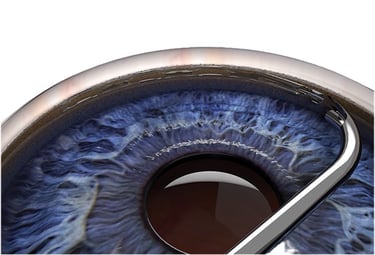
Minimally invasive glaucoma surgery (MIGS)

Ophthalmic diagnostic testing instruments
Femtosecond lasers for ophthalmology
Cardiac mapping & ablation





Implantable drug delivery
Spinal cord stimulation
Infectious disease diagnostic testing
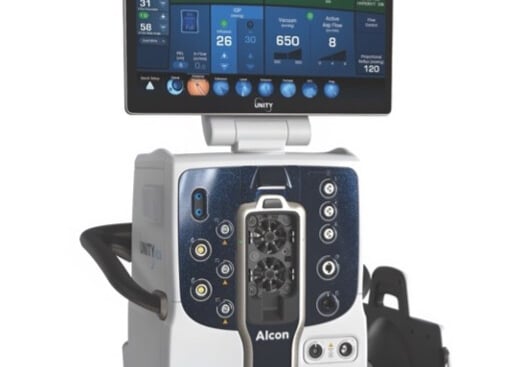
Robotic surgery systems
Insulin pumps
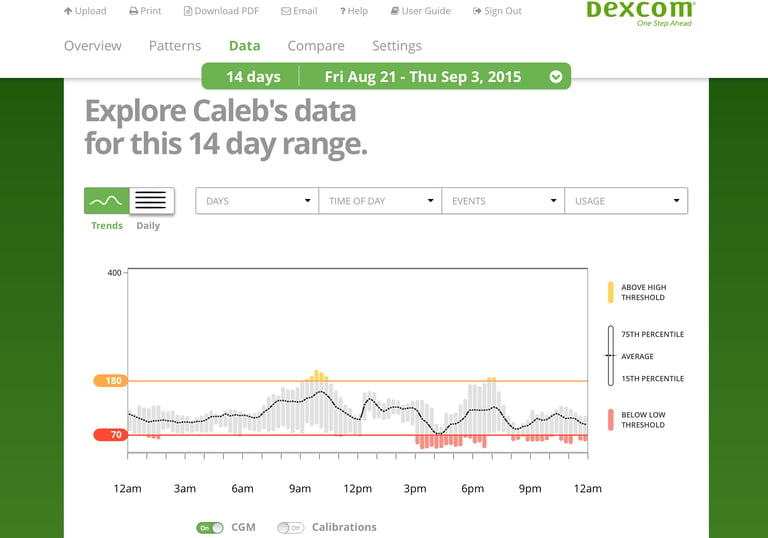
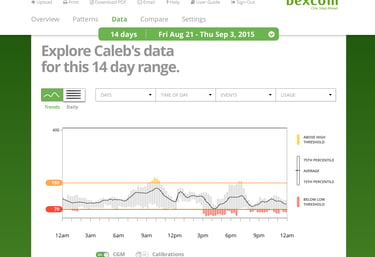
Continuous glucose monitoring systems
Deep brain stimulation
Point-of-care diagnostic testing
Tissue microdebrider
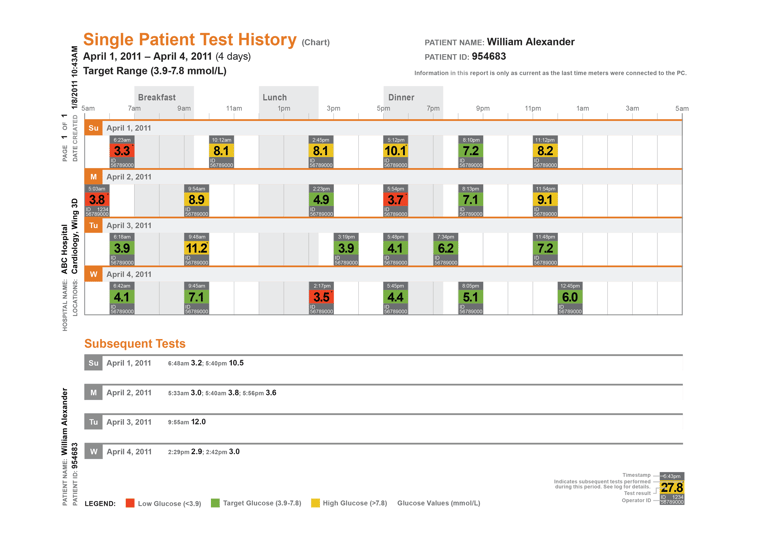
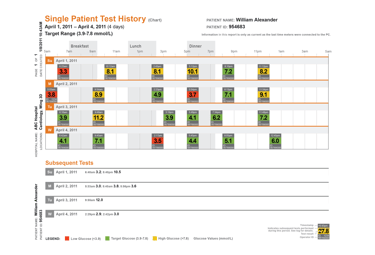
Hospital blood glucose management
Vitreo-retinal & cataract surgery systems
Orthopedic drillsaws
Blood glucose management software
Sleep apnea devices
Intraocular pressure measurement

